Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
27 May, 2020
Highlights
Elective and non-essential medical procedures are on an indefinite hold in many places. Simultaneously, essential medical services are in high demand, and likely to remain in demand for the near future. This dynamic creates winners and losers among Health Care device manufacturers and distributors.
Investors can identify potential opportunities in the Health Care Equipment and Services subsector by analyzing 510(k) premarket notifications, which are filings required by the U.S. Food and Drug Administration (FDA) for any company seeking to market a medical device in the United States.
One example of the aforementioned dichotomy is Cutera Inc. versus Vapotherm Inc. (Figure 1). The former specializes in devices used in cosmetic surgery and has declined 48% from February 28 to May 19. In contrast, Vapotherm, which specializes in products used to treat respiratory distress, has seen a 176% return over the same period. Vapotherm’s oxygen controller was awarded ‘breakthrough device’ status by the FDA on April 9.
Figure 1. Performance of Vapotherm, Inc. and Cutera, Inc. alongside the S&P 500 Total Return and the Health Care Sector SPDR (XLV)
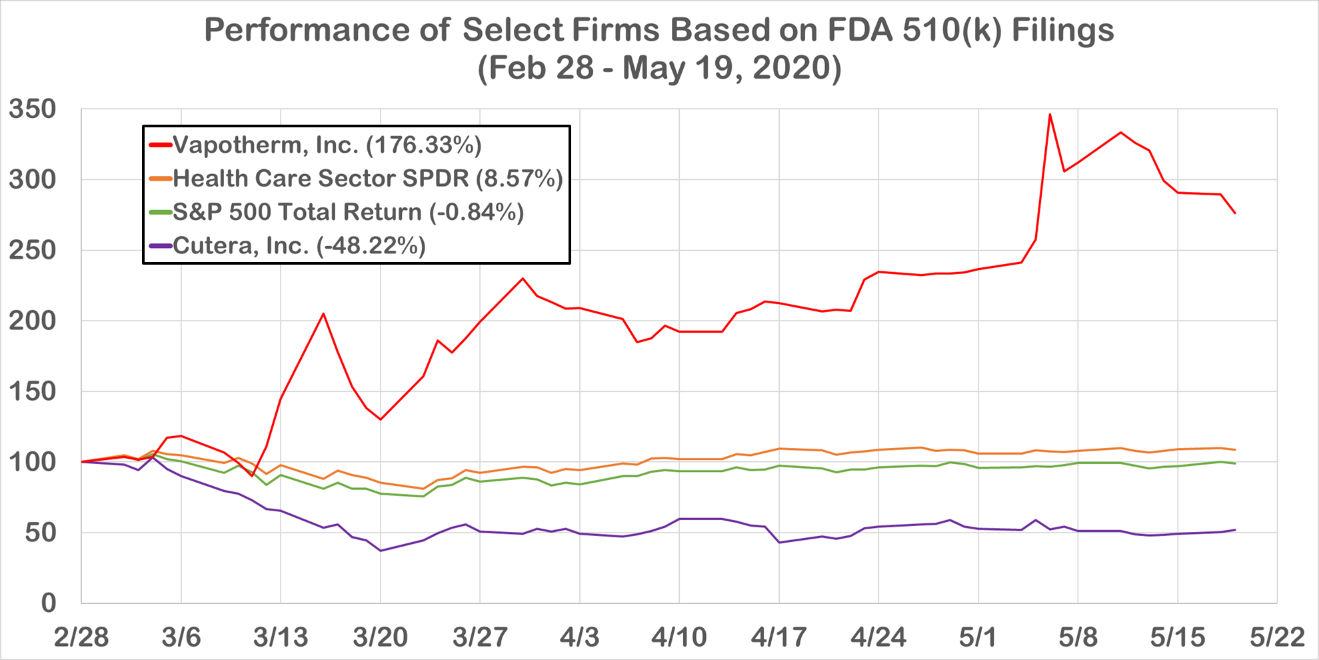
Source: S&P Global Market Intelligence Quantamental Research. Data as of May 20, 2020.
Please access the complete list of Quantamental Research Briefs for the latest on COVID-19’s impact.
Download The Full Report
Research
Research
Products & Offerings
Segment
